- Accueil
- 1-3
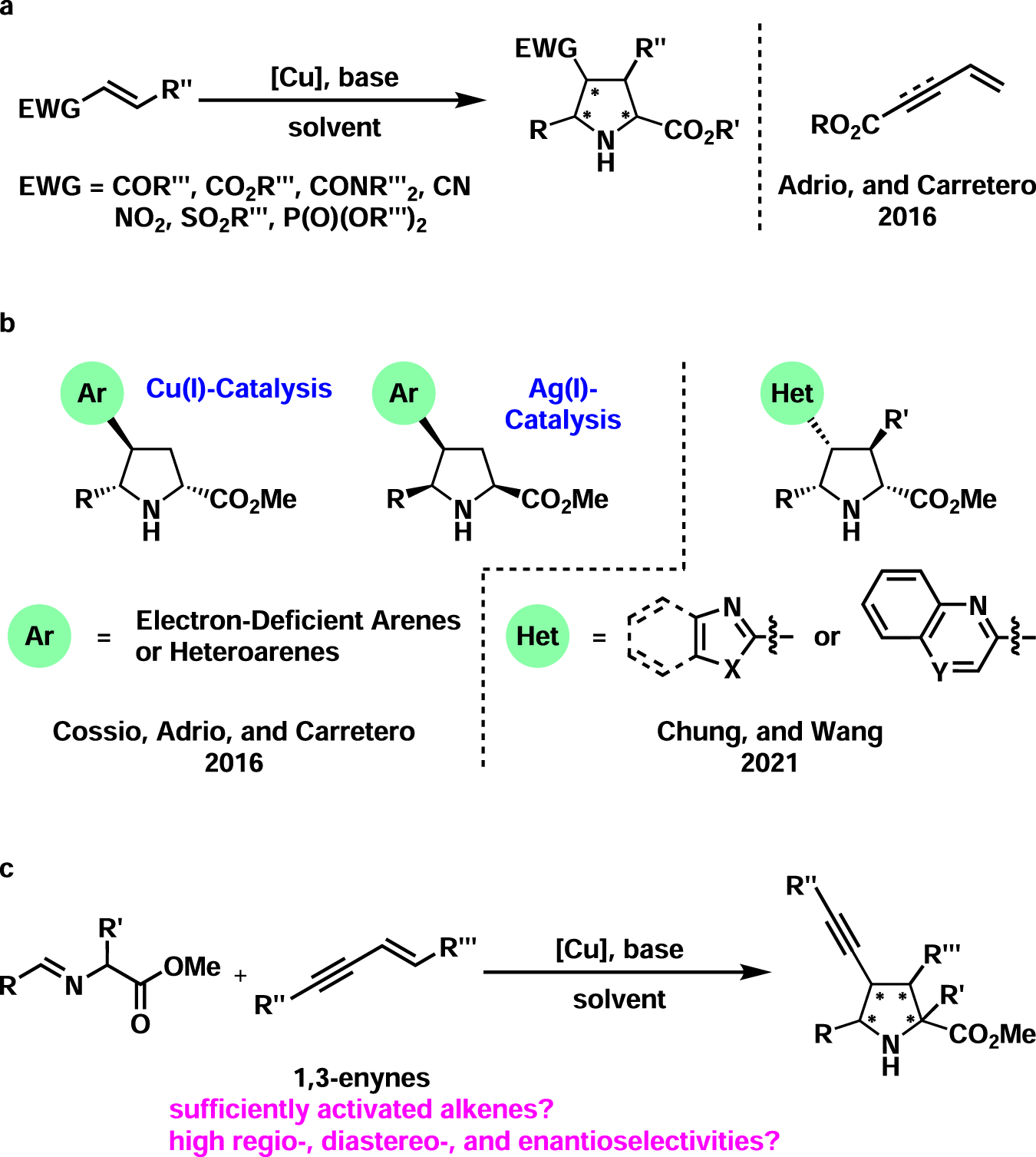
- Copper(I)-catalyzed asymmetric 1,3-dipolar cycloaddition of 1,3-enynes and azomethine ylides
Copper(I)-catalyzed asymmetric 1,3-dipolar cycloaddition of 1,3-enynes and azomethine ylides
4.9 (746) · € 28.00 · En Stock

Recent advances in the enantioselective 1,3-dipolar cycloaddition of azomethine ylides and dipolarophiles - ScienceDirect

Cao's regiodivergent boroprotonation of trifluoromethyl-substituted

成果及论文 - 邓卫平课题组

Kinetic Resolution of Alkylidene Norcamphors via a Ligand-Controlled Umpolung-Type 1,3-Dipolar Cycloaddition. - Abstract - Europe PMC

Copper(I)-catalyzed asymmetric 1,3-dipolar cycloaddition of 1,3-enynes and azomethine ylides

Copper(I)-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides with Fluorinated Imines: The Expanded Scope and Mechanism Insights

Kinetic Resolution of Alkylidene Norcamphors via a Ligand-Controlled Umpolung-Type 1,3-Dipolar Cycloaddition. - Abstract - Europe PMC
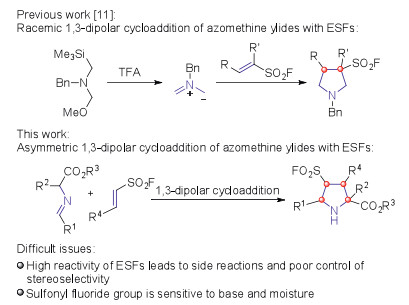
Cu-catalyzed endo-selective asymmetric 1,3-dipolar cycloaddition of azomethine ylides with ethenesulfonyl fluorides: Efficient access to chiral pyrrolidine-3-sulfonyl fluorides

Kinetic Resolution of Alkylidene Norcamphors via a Ligand-Controlled Umpolung-Type 1,3-Dipolar Cycloaddition. - Abstract - Europe PMC

Copper(I)-catalyzed asymmetric 1,3-dipolar cycloaddition of 1,3-enynes and azomethine ylides

Strategies for synthesis of chiral exocyclic allenes. a-c Previous

Recent advances on copper-catalyzed asymmetric synthesis and their potential biological applications - ScienceDirect

Copper(I)-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides with Fluorinated Imines: The Expanded Scope and Mechanism Insights