SalivaDirect™ COVID-19 Testing Process < Pathology
4.9 (457) · € 37.50 · En Stock
Our quick and affordable saliva-based COVID-19 test developed by Yale scientists has received FDA Emergency Use Authorization. The Pathology Clinical Molecular
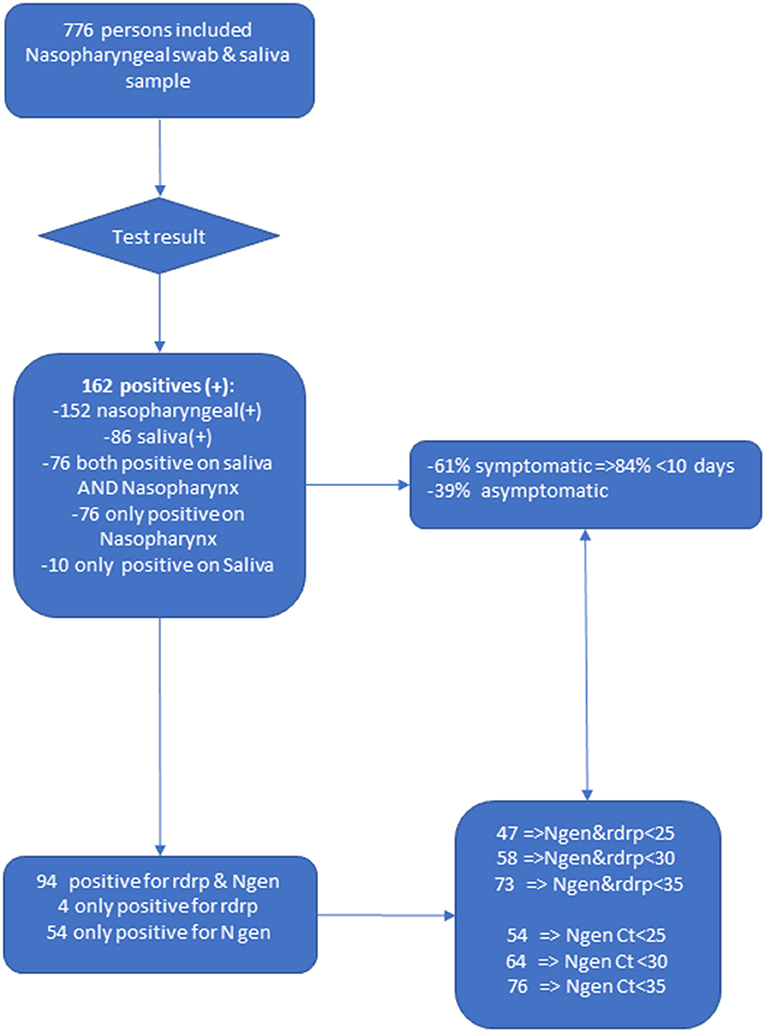
Frontiers Prospective Comparison of Saliva and Nasopharyngeal Swab Sampling for Mass Screening for COVID-19

SalivaDirect, Inc. (@saliva_direct) / X

Yale designates labs in three states to provide SalivaDirect™ COVID-19 test

SaliVISION: a rapid saliva-based COVID-19 screening and diagnostic test with high sensitivity and specificity

COVID Test Direct Diagnostics
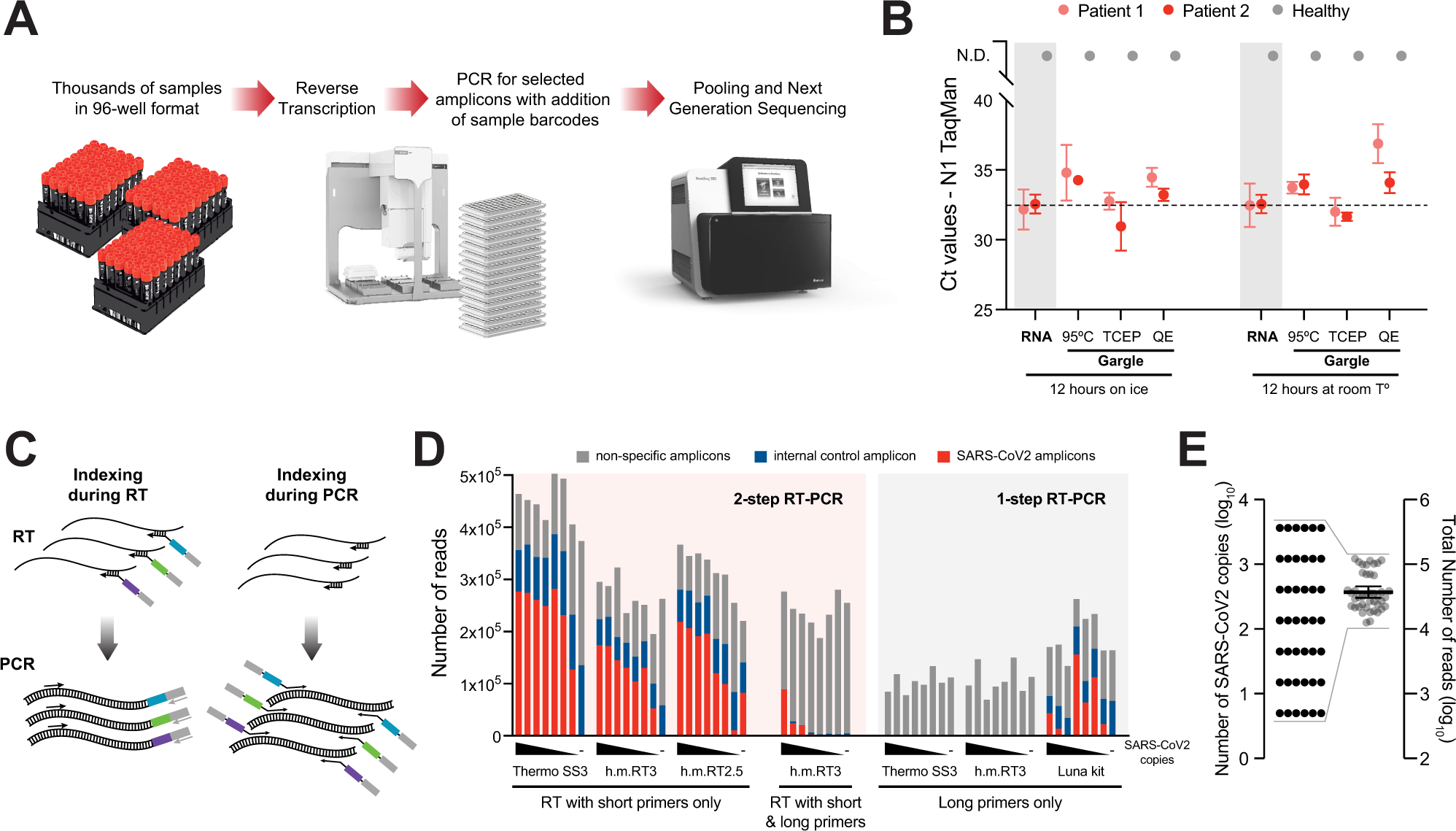
Multiplexed detection of SARS-CoV-2 and other respiratory infections in high throughput by SARSeq

Protocols < SalivaDirect™
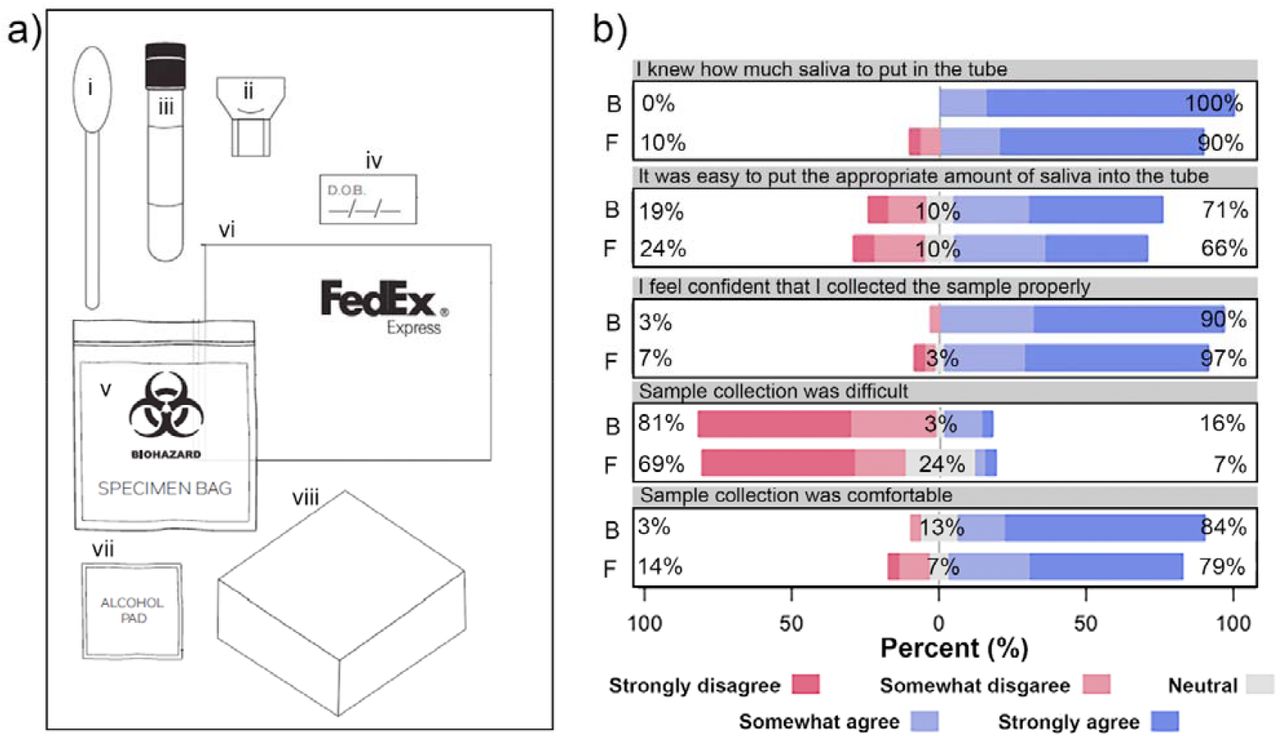
Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics

COVID Test Direct Diagnostics

About SalivaDirect < SalivaDirect™

SaliVISION: a rapid saliva-based COVID-19 screening and diagnostic test with high sensitivity and specificity