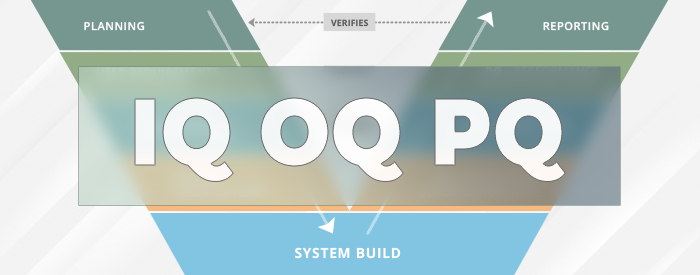
What is IQ OQ PQ in Software Validation?
5 (135) · € 30.00 · En Stock
If you work in a regulated industry (FDA), you know about IQ, OQ, PQ testing—let CSols Inc. de mystify these scripts on your behalf.
The OQ/PQ Validation Protocol is used to document the system's ability to meet the functional requirements specifications (OQ) and user requirements

CSV Template - Validation Protocol - OQ or PQ

The Complete Guide to Computer System Validation: IQ, OQ, PQ, – Blue Mountain

What is Qualification Protocol (IQ/OQ/PQ/IFQP), Its Necessity & When re-qualification performed in CSV? – Be Innov@tive

What is IQ OQ PQ in Software Validation?
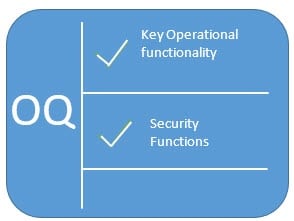
What Are IQ OQ PQ, The 3 Q's Of Software Validation Process

FDA Software Validation - 2022 Guide, Checklist & Template
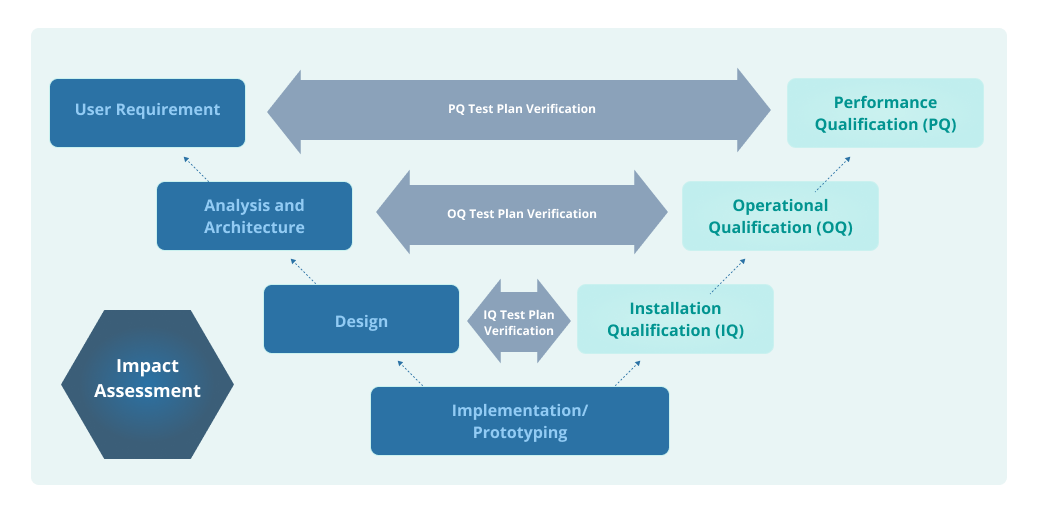
A Guide to IQ, OQ, and PQ in FDA-Regulated Industries

IQ, OQ, PQ: what's needed for equipment validation in life sciences?
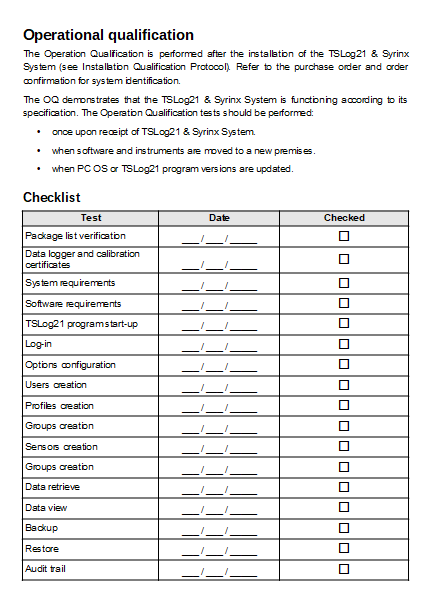
IQ_QQ_PQ_Protocols