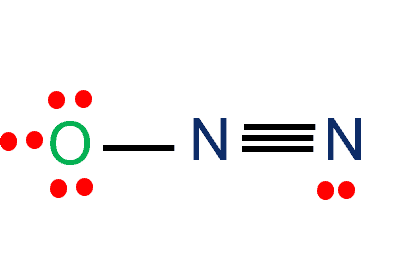
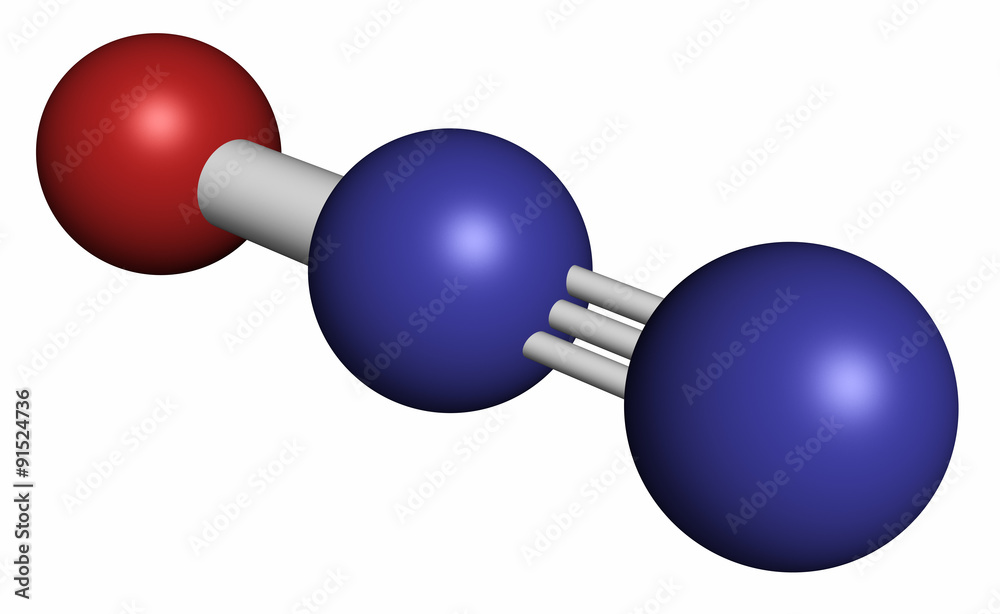
N2O lewis structure, molecular geometry, bond angle, hybridization
4.5 (531) · € 22.99 · En Stock
Nitrous oxide (N2O) Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, hybridization, formal charges, polar vs non-polar

Which of the following molecules have the same geometries? a. CO2 and BeH2 b. SF4 and CH4 c. CO2 and NO2 d. N2O and NO2

Comparison of the six possible N2O-bound models The models are

Carbon tetrachloride CCl4 lewis dot structure, molecular geometry, polar or nonpolar, Bond angle

N2O Lewis Structure, Geometry - Kemicalinfo

Solved a) Identify the number of valence electrons used in
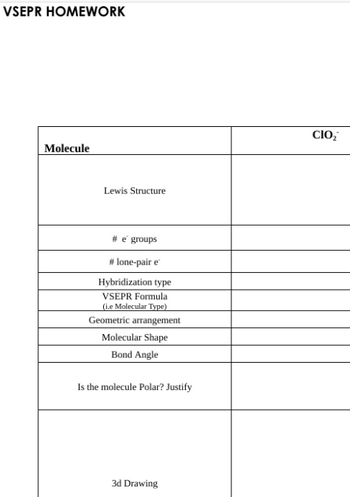
Answered: Molecule Lewis Structure #e groups…

Chem 105 Chapter 5 Homework Flashcards

Which two of the following molecules have the same molecular geometry? Please show your work (Lewis structures and 3D molecular shapes) and explain your reasoning. a. N2O b. Se2O c. CO2
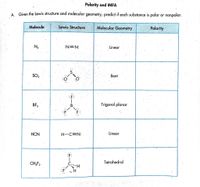
Answered: Polarity and IMFA A. Given the Lewis…
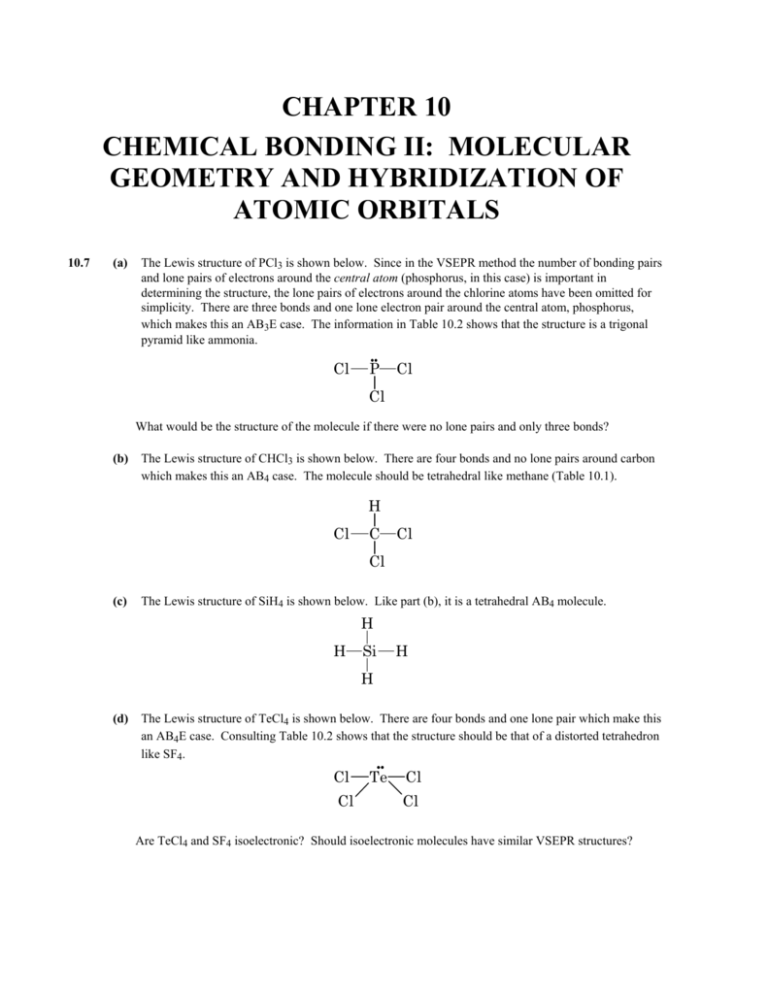
chapter 10 chemical bonding ii
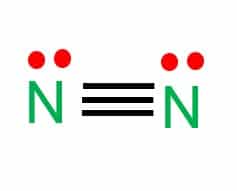
N2 Lewis Structure Hybridization & Molecular Geometry - What's Insight
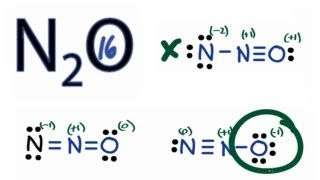
N2O Lewis, Shape, Hybridization, Polarity, and more.